Masses of atoms, molecules and ions


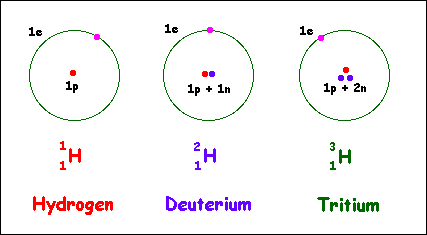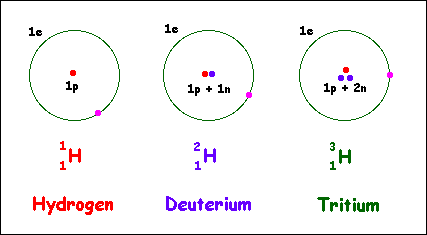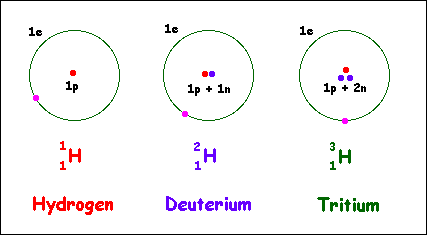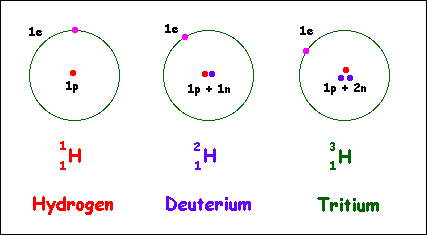
PROTIUM (HYDROGEN)
- the simplest atom of the element
DEUTERIUM
- an atom with 1 neutron
TRITIUM
- an atom with 3 neutrons

THE MASSES OF ATOMS
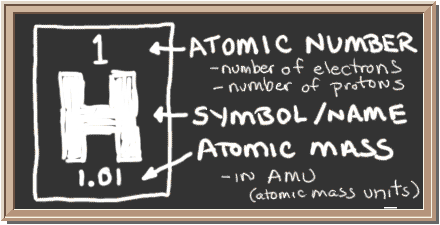
A mass of an atom is known by the ATOMIC NUMBER, Ar.
This represents the number of protons (+-charge) in a nucleas, and the number of electrons (- charge) (in a neutral atom).
So the number of protons/electrons are equal in number, carrying no overall charge due to protons and electrons balancing each other.
*Number of protons are usually rounded off
For example
A hydrogen atomic number is 1 (smallest number in the box), having 1 numbers of protons and 1 of electrons, both balancing one another.
E.G. HYDROGEN ISOTOPES
This is the types of isotope in a hydrogen. ISOTOPES are atoms of a single element, with the same number of protons but a different number of neutrons in the neuclei (diffferent masses). In this diagram shows :
TYPES OF ISOTOPES
From this example, this shows that isotopes have different masses!
This types of isotopes shows different masses when the number of neutrons is increased.
Finding masses of molecule and ions

Mass of ION
An ion (a group of atoms) uses its original Ar, having the same mass as the atom which is made.
A mass for an ion is called RELATIVE FORMULA MASS, Mr.
Mass of MOLECULES
A molecule's mass would be greater than its original Ar, due to two or more atoms held together.
For a subctance to be made into a molecule,its mass is reffered to RELATIVE MOLECULAR MASS, Mr.



Example of RELATiVE FORMULA MASS

Example of RELATIVE MOLECULAR MASS
Carbon Dioxide gas is known as a molecule. It has one molecule of carbon and 2 molecules of oxygen. So its mass is 44. [ 12+2(16) = 44 ]
